Smart Intrathecal Prescription
IT prescription software: automated dose calculations, error reduction, integrated pharmacy workflow. SynchroMed II pumps, morphine/ropivacaine/ziconotide management. CE certification in progress, HDS, HAS compliant.
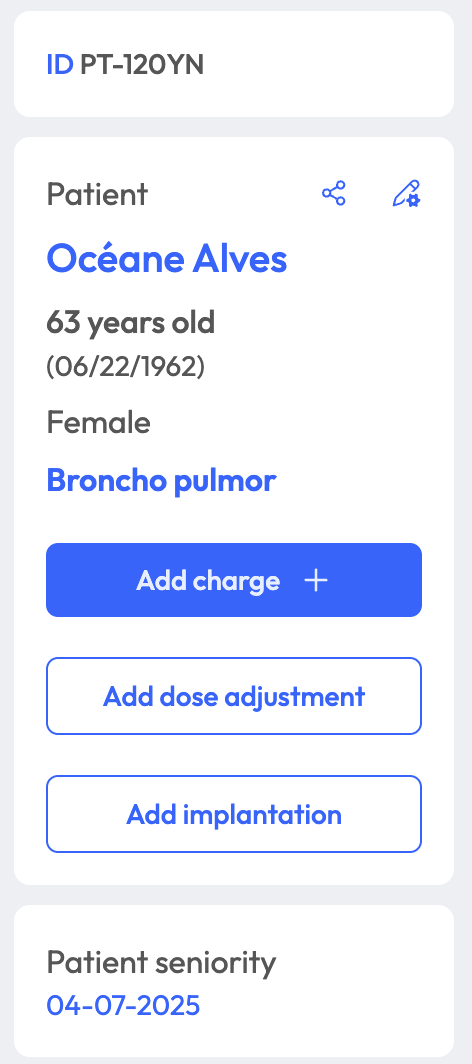
Prescribe intrathecal therapies with a structured, safe interface. Integrate drug, concentration, pump type, and administration schedule with automatic recommandations.
Support for both continuous and sequential infusions, with precise modulation by phase, dose, volume and flow.
Collaborative validation workflows with pharmacists. Includes dose rounding suggestions, dilution constraints, and traceability logs.
FHIR/HL7 standards ensure seamless EMR integration. CE class I certification in progress (expected January 2026). Anonymized data export to FAIR research registries.
Prescription Made Smarter
Thalivia is a web-based solution designed for clinicians practicing intrathecal analgesia. Developed with medical experts, it offers a secure, structured way to prescribe, validate, and share complex treatment plans.
Precision at Every Step
- Dose Calculation: Automated based on drug, concentration, pump type, and desired flow.
- Sequential and Continuous Programming: Phase-based modulation with real-time feedback.
- Safety Checks: Recommandations for incompatible doses, volumes, and flows.
Workflow Integration
For Prescribers
- Create, update and manage patient records, treatment facilities, pump settings (“charges”) and prescription plans
- Share cases and collaborate with peers
- MFA & secure access (HDS, GDPR compliant)
For Pharmacists (Entreprise account only)
- Accept/modify/reject prescriptions
- Enforce local preparation rules
- Generate printable prep sheets and labels
For Institutions (Entreprise account only)
- SSO (OpenID), patient lookup (IHE PIX/PDQ)
- Export in FHIR format
- Support for national drug registries (BDPM, AEMPS…)
Trusted and Supported
- Expert-backed: Co-developed with Dr. Denis Dupoiron (Head of Pain Dept., Institut de Cancérologie de l’Ouest), laureate of the Prix Axel Kahn 2023 and leading European expert in intrathecal analgesia.
- Clinically validated: Based on 12+ years of clinical data and 50+ peer-reviewed publications in pain medicine.
- Hosted in France: HDS-compliant infrastructure with daily backups and security audits.
- Regulatory compliant: CE Class I medical device certification in progress (expected January 2026), GDPR compliant, aligned with HAS and SFETD guidelines.
With Thalivia, prescribing intrathecal treatments is designed to support safety, efficiency, and clinical decision-making—while aligning with clinical standards and regulatory needs.
Discover how Thalivia supports intrathecal prescriptions—designed for safety, efficiency, and full interoperability.